Thermodynamics is a science dealing with energy and its transformation. The basic of thermodynamics is deals with the equilibrium and feasibility of a process.
Table of Content
Table of Content
- What is Thermodynamics?
- What is the importance of thermodynamics?
- Laws of Thermodynamics :
- Different Approaches to thermodynamics
- What is System in thermodynamics?
- Surroundings
- Isolated System in thermodynamics
- Closed System in thermodynamics
- Open System in thermodynamics
What is Thermodynamics?
It deals with the equilibrium and feasibility of a process.Thermodynamics is a science dealing with energy and its transformation.
It also deals with relations between heat and work and the properties of a system.
What is the importance of thermodynamics?
The main task of an engineer is to ensure optimum use of the resources-space, time, energy, and matter. Therefore, an engineer deals with the design and development of new processes and with the improvement of the existing processes. Before undertaking an expensive project, an engineer must know the answers to the following types of questions.
- Is the proposed chemical reaction or physical process possible?
- Does the reaction process go to completion, or does it proceed to a certain extent only beyond which it cannot proceed?
- What factors govern the extent of reaction or equilibrium?
- How much energy is required for the process to take place?
- What is the maximum efficiency of a heat engine or the maximum coefficient of performance of a refrigerator?
- Thermodynamics provides answers to the above types of questions.
Also, ReadLaws of Thermodynamics :
Zeroth law of thermodynamics
It deals with thermal equilibrium state and basis for measure temperature. It also gives the concept of Isotherms.
The first law of thermodynamics
It tells about the conversation of energy and introduces the concept of internal energy.
The second law of thermodynamics
It dictates the limits of converting internal energy into work and introduces the concept of entropy. It also gives ide whether a particular process is feasible or not.
Third law of thermodynamics
It provides a datum for the measurement of entropy.
- The laws of thermodynamics cannot be directly proved. They were deduced from experimental results through logical reasoning.
- The validity of the laws of thermodynamics rests upon the fact that, to date, no experimental evidence is available to disprove them.
Different Approaches to thermodynamics
There are two different approaches to the study of thermodynamics. They are macroscopic and microscopic.
Macroscopic
1. The structure of matter is not considered.
2. Only a few variables are used to describe the state of matter.
3. The values of these variables can be measured.
4. Classical thermodynamics adopts the macroscopic approach.
Microscopic
1. Knowledge of the structure of mater is essential.
2. A large number of variables are needed to describe the state of matter.
3. The values of these variables cannot be measured.
4. Statistical thermodynamic adopts the microscopic approach.
In the macroscopic approach, fluids are treated as continuous rather than made up of several individual particles.
The macroscopic approach is not valid in situations where very few molecules are involved or where the the behavior of individual particles is sought.
As an illustration, consider the pressure of gas exerts on the walls of its container. This pressure results from the change of momentum of the molecules as they collide with the wall. However, from a macroscopic point of view, we are not concerned with the action of the individual molecules but with the time average force on a given area, which can be measured by a pressure gauge. In fact, macroscopic observations are entirely independent of assumptions regarding the nature of the matters.
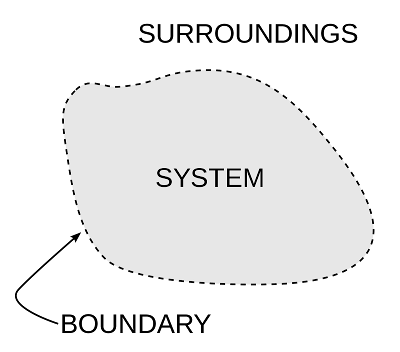
What is System in thermodynamics?
In the scientific analysis, it is essential to clearly identify the subject matter of analysis on which we focus our attention. In mechanics, a part of the body is isolated from the rest of the universe, and we draw a free body diagram and analyze the motion by applying Newton's laws of motion. Similarly, in thermodynamics, also we identify the subject of analysis by term thermodynamics system or System for Simplicity to specify the matter on which we focus our attention.
A system is a definite quantity of matter bounded by some surface, which separates it from the rest of the world.
The boundary surface may be real or imaginary. It may change in shape and size. A System may be very simple like a gas contained in a cylinder, or it may be complex like a thermal power plant. The choice of a system may differ from the person performing the analysis. Sometimes the System is also referred to as control mass.
Surroundings
The combination of matter and space external to the System constitutes the surroundings.
For all practical purposes, that part of the Surroundings where the effects due to interaction between a system and its surroundings are not detectable and need not be considered.
A system can exchange energy in the form of work and heat with its surroundings. A system that is enclosed by an adiabatic boundary cannot exchange energy as heat with its surroundings.
Isolated System in thermodynamics
A system that is enclosed by a rigid and adiabatic boundary cannot exchange energy either as heat or work with its surroundings. Such a system is called an isolated system.
In this System, neither the mass to the energy crosses the boundaries of the System.
Closed System in thermodynamics
The closed system is one in which the boundaries are closed so that no substance be may enter or leave the System.
In such a system, the mass of the substance within the system remains constant.
A transfer of energy may, however, take place at the boundaries.
Open System in thermodynamics
The open System is one, the boundaries of which are not closed but have one or more openings through which mass transfer may also take place in addition to energy transfer.
If the rate of mass and energy transfer concerning time is constant, the System is known as a steady flow system.
Also, Read
Thermodynamics Books | Thermal Engineering Books